Above the Critical Temperature a Substance
3108 C 5840 K 102 atm 10300 kPa Caesium. So this is the definition for the critical temperature of the substance and it is represented by TC.
What Is Critical Temperature Quora
At slightly above the critical temperature 310 K in the vicinity of the critical pressure the line is almost vertical.

. D can never be a solid again even after it is cooled. The temperature which above a substance can not exist as a liquid no matter how much pressure is applied. He critical temperature of a substance is the The critical temperature of a substance is the temperature above which the compound decomposes.
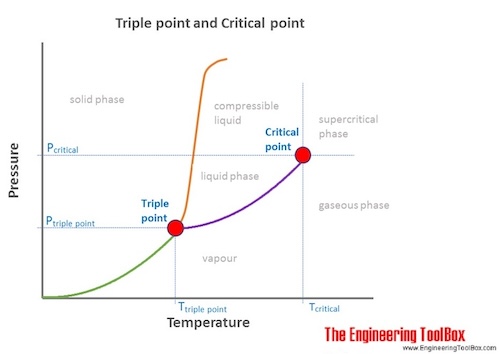
At the critical point 3041 K and 738 MPa there is no difference in density and the two phases become one fluid phase. The combination of the critical temperature and critical pressure of a substance is its critical point. At Curie temperature the ferromagnetic materials get converted into.
Every substance has a critical temperature. In the supercritical environment only one phase exists. Above the critical temperature and pressure a substance exists as a dense fluid called a supercritical fluid which resembles a gas in that it completely fills its container but has a density comparable to that of a liquid.
A substance can a guest engaged only in Only in guess he is guess heres state. C will slowly crystallize into its most stable form. If the temperature is below critical temp and the pressure is also below its critical value then there is a possibility that we see an equilibrium between the liquid and gaseous states.
The critical temperature of carbondioside is 311C. At a certain temperature a ferromagnetic material becomes paramagnetic. The correct answer among the choices given is option C.
What is this temperature called. The critical temperature of a substance can therefore be defined as the pressure that must be applied to a substance in order. At temperatures above the critical temperature of a substance it cannot be liquified with the application of any amount of pressure.
The statement that the entropy of a pure substance in complete theromodynamic equilibrium becomes zero at the absolute zero of temperature is known as. A substance held at a temperature above its critical temperature A always decomposes. At the critical temperature they cannot longer be liquified.
Thus above the critical temperature a gas cannot be liquified by pressure. B cannot be condensed at this temperature regardless of the pressure applied. 1224 C 1508 K 481 atm 4870 kPa Ammonia NH 3 1324 C 4055 K 1113 atm 11280 kPa R-134a.
If the room temperature is 347C then carbondioxide at room temperature is. 10106 C 37421 K 4006 atm 4059 kPa R-410A. Any substance above its critical temperature exists as.
728 C 3459 K 4708 atm 4770 kPa Bromine. The name associated with the above statement is. Select the correct answer below.
View the full answer. The Curie temperature for a ferromagnetic substance is 3 0 0 K. When one of them is above their critical value it is impossible to change the state of the substance from gas to liquid by merely changing the other parameter.
This fluid is any substance at a temperature and pressure above the critical point here the phases of the liquid and the gas do not exist. Temperature at which sublimation occurs. Temperature at which all three phases can exist in equilibrium.
At the critical point the gas and the liquid phases become identical and the visible boundary between the two phases vanishes. Highest temperature at which the liquid phase can exist in equilibrium with the gas phase. Solve any question of States Of Matterwith-.
100 22 ratings Critical Temperature is defined as the maximum temperature at which a. Above the critical pressure a substance is a supercritical fluid. Above the critical temperature a substance.
6 at 4 5 0 K temperature then find out Curie constant. Substance Critical temperature Critical pressure absolute Argon. The pressure exerted by a vapor in equilibrium with its liquid at a given temperature.
Question Above the critical temperature a gas will liquefy. 8 rows The highest temperature of a substance at which it can be condensed and remain in a liquid state. The critical temperature of a substance is the temperature above which a substance can exist only in gaseous state.
Okay So at the temperature above critical temperature the face of the gas will be face of the substance will be gaseous face only. The critical temperature of a substance can be defined as the highest temperature at which the substance can exist as a liquid. A supercritical fluid is a substance above its critical temperature and pressure.
Hence Option B is the correct answer. The lowest pressure under which a substance can exist as a liquid at the critical temperature. If magnetic susceptibility of the substance is 0.
The pressure required to liquify a substance vapor at its critical temperature. Physical chemistry The temperature of the liquid-vapor critical point that is the temperature above which the substance has no liquid-vapor transition. Soobee72pl and 23 more users found this answer helpful.
Kridəkəl temprəchər agriculture The temperature below which a plant cannot grow. At the highest possible pressure permanently O at no pressure value depends on the substance. Any substance above its critical temperature exists as.
What is the critical pressure. At temperatures above the critical temperature the substance in question in its vapourgaseous state can no longer be liquified regardless of the amount pressure applied to it.

Critical Temperature Temperature Vs Pressure Graph Examples

Supercritical Fluid Critical Point Occurs Under Conditions Such As Specific Values Of Pressure And Temperature At Which No Phase Boundaries Exist Therefore Th
Critical Temperatures And Pressures For Some Common Substances
No comments for "Above the Critical Temperature a Substance"
Post a Comment